Search This Blog
Hllo students hope u do'in good , welcome and search your query here
Featured Post
- Get link
- X
- Other Apps
structure of atom class 9 solutions NCERT CBSE science
structure of atom class 9 solutions NCERT CBSE science
1. Compare the properties of electrons, protons, and neutrons.
Answer: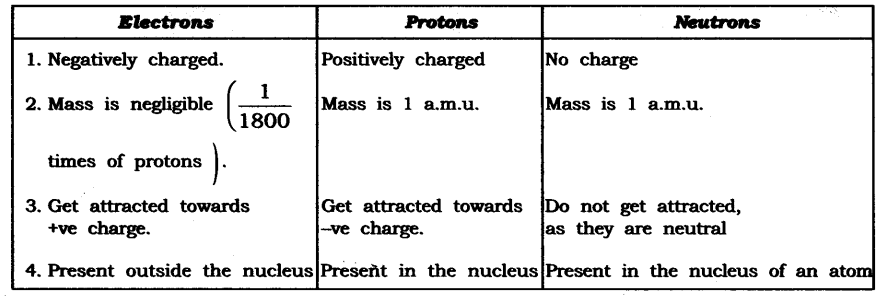
Question 2. What are the limitations of J.J. Thomson’s model of the atom?
Answer: According to J.J. Thomson’s model of an atom, the electrons are embedded all over in the positively charged spheres. But experiments done by other scientists showed that protons are present only in the center of the atom and electrons are distributed around it.
Question 3. What are the limitations of Rutherford’s model of the atom?
Answer: According to Rutherford’s model of an atom the electrons are revolving in a circular orbit around the nucleus. Any such particle that revolves would undergo acceleration and radiate energy. The revolving electron would lose its energy and finally fall into the nucleus, the atom would be highly unstable. But we know that atoms are quite stable.
Question 4. Describe Bohr’s model of the atom.
Answer: Bohr’s model of the atom
(1) Atom has a nucleus in the center.
(2) Electrons revolve around the nucleus.
(3) Certain special orbits known as discrete orbits of electrons are allowed inside the atom.
(4) While revolving in discrete orbits the electrons do not radiate energy.
(5) These orbits or shells are called energy levels.
(6) These orbits or shells are represented by the letters K, L, M, N or the numbers n = 1, 2, 3, 4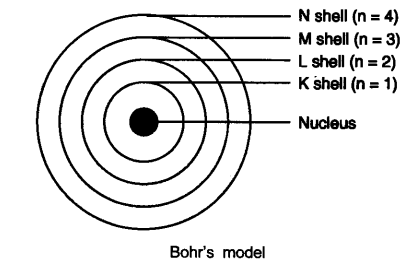
Question 5. Compare all the proposed Bohr’s models of an atom given in this chapter.
Answer: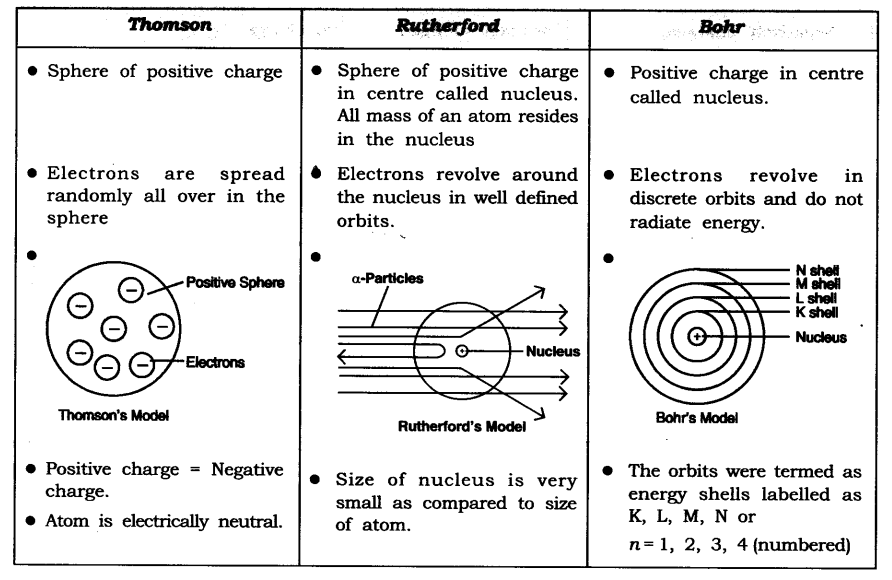
Question 6. Summarise the rules for writing the distribution of electrons in various shells for the first eighteen elements.
Answer: The rules for writing of distribution of electrons in various shells for the first eighteen elements are:
(i) The maximum number of electrons present in a shell is given by the formula-2 n2
∵ n = orbit number i.e., 1, 2, 3
∵ Maximum number of electrons in different shells are:
K shell n = 1 2n2 => 2(1)2 = 2
L shell n = 2 2n2 => 2(2)2 = 8
M shell n = 3 2n2 => 2(3)2 = 18
N shell n = 4 2n2 => 2(4)2 = 32
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons are not accommodated in a given shell unless the inner shells are filled. (Shells are filled step-wise).
Question 7. Define valency by taking examples of silicon and oxygen.
Answer: Valency is the combining capacity of an atom.
Atomic number of oxygen = 8 Atomic number of silicon = 14 K L M
Electronic configuration of oxygen = 2 6 –
Electronic configuration of silicon =2 8 4
In the atoms of oxygen, the valence electrons are 6 (i.e., electrons in the outermost shell). To fill the orbit, 2 electrons are required. In the atom of silicon, the valence electrons are 4. To fill this orbit 4 electrons are required.
Hence, the combining capacity of oxygen is 2 and silicon is 4.
i.e., Valency of oxygen = 2
Valency of silicon = 4
Question 8. Explain with examples:
(i) Atomic number (ii) Mass number,
(iii) Isotopes and (iv) Isobars.
Give any two uses of isotopes.
Answer: (i) Atomic number: The atomic number of an element is equal to the number of protons in the nucleus of its atom. e.g., Oxygen has 6 protons hence atomic no. = 6.
(ii) Mass number: The mass number of an atom is equal to the number of protons and neutrons in its nucleus.
Nucleons = number of protons + number of neutrons Example: Protons + Neutrons = Nucleus = Mass number 6 + 6 = 12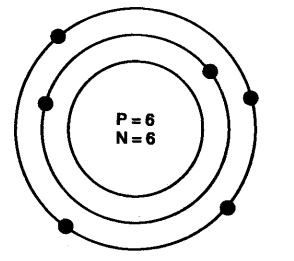
(iii) Isotopes: Isotopes are atoms of the same element which have a different mass number but the same atomic number.![]()
(iv) Isobars: Isobars are atoms having the same mass number but different atomic numbers.![]()
Both calcium and argon have the same mass number but a different atomic number.
Two uses of isotopes are:
(i) An isotope of iodine is used in the treatment of goiter.
(ii) An isotope of uranium is used as a fuel in nuclear reactors.
Question 9. Na+ has completely filled K and L shells. Explain.
Answer: Sodium atom (Na), has atomic number =11
Number of protons =11
Number of electrons = 11
Electronic configuration of Na = K L M – 2 8 1
Sodium atom (Na) loses 1 electron to become stable and form Na+ ion. Hence it has completely filled K and L shells.
Question 10. If a bromine atom is available in the form of say, two isotopes 7935Br (49.7%) and 8135Br (50.3%), calculate the average atomic mass of the bromine atom.
Answer: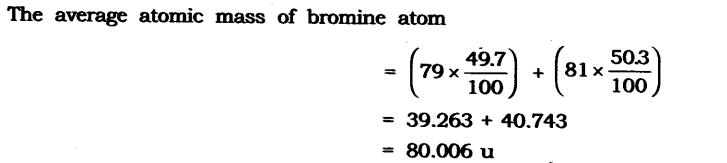
Question 11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168X and 188X in the sample?
Answer: Let the percentage of 168X be x and the percentage of 168X be 100 – x.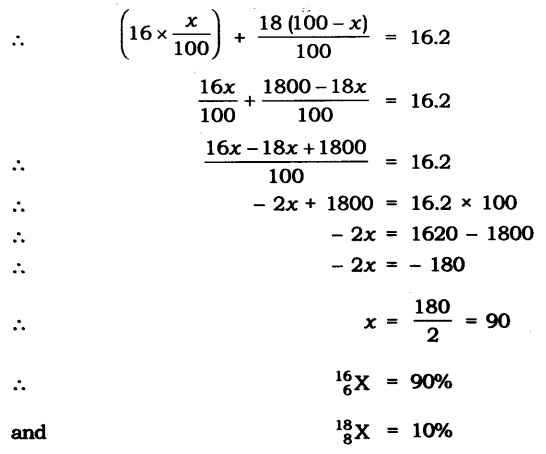
Question 12. If Z = 3, what would be the valency of the element? Also, name the element.
Answer: Z = 3, (i.e, atomic number —> z)
∴ Electronic configuration = 2, 1
Valency = 1
The name of the element is lithium.
Question 13. Composition of the nuclei of two atomic species X and Y are given as under
X – Y
Protons =6 6
Neutrons = 6 8
Give the mass number of X and Y. What is the relation between the two species?
Answer: Mass number of X = Protons + Neutrons
= 6 + 6 = 12
Mass number of Y = Protons + Neutrons = 6 + 8 = 14
As the atomic number is same i.e., = 6.
[atomic number = number of protons].
Both X and Y are isotopes of the same element.
Question 14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of the proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer: (a) False (b) False
(c) True (d) False
Put a tick against correct choice and cross (x) against the wrong choice in questions 15, 16, and 17.
Question 15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus (c) Proton
(b)Electron (d)neutron
Answer: (a) Atomic nucleus
Question 16. Isotopes of an element have
(a) the same physical properties (c) the different number of neutrons
(b)a different number of neutrons (d) different atomic numbers.
Answer: (c) different number of neutrons
Question 17. Number of valence electrons in Ct ion are :
(a) 16 (b) 8
(c) 17 (d) 18
Answer: (b) 8
Question 18. Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8 (b) 8, 2, 1
(c) 2, 1, 8 (d) 2, 8, 1
Answer: (d) 2, 8, 1
Question 19. Complete the following table.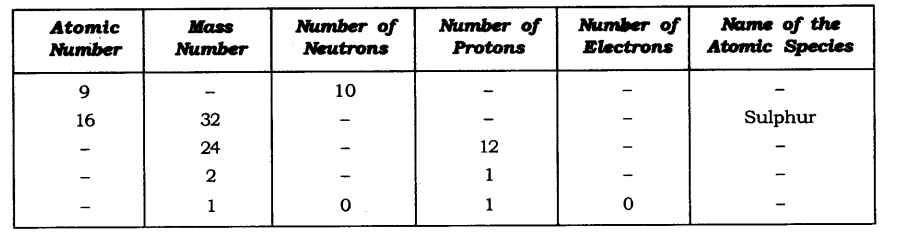
Answer: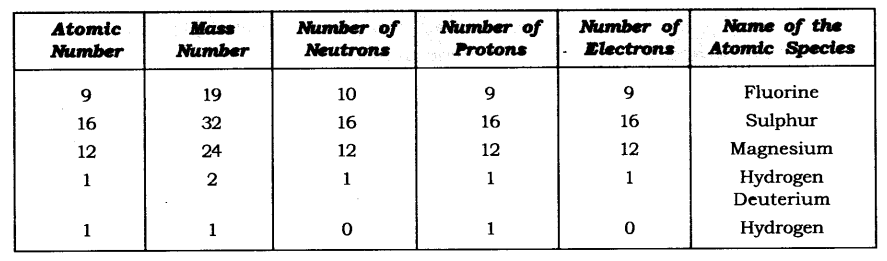
Popular Posts
Allen test series 2020 free pdf download for neet
- Get link
- X
- Other Apps
the living world exemplar solutions NEET NCERT
- Get link
- X
- Other Apps
Allen Test Series For Neet 2017 Pdf Free Download
- Get link
- X
- Other Apps
living world class 11th NCERT solutions CBSE
- Get link
- X
- Other Apps
DC PANDEY for neet preparation pdf full book pdf
- Get link
- X
- Other Apps
strategies for enhancement in food production solutions NCERT CBSE class 12
- Get link
- X
- Other Apps
Allen Test Series For Neet 2019 Pdf Free Download
- Get link
- X
- Other Apps
Allen Test Series For Neet 2018 Pdf Free Download
- Get link
- X
- Other Apps
the fundamental unit of cell class 9 solutions NCERT CBSE
- Get link
- X
- Other Apps

Comments
Post a Comment
Hello ✋ welcome ,I hope u get something today